
NHIA to HHS: Make Medicare Payment for COVID-19 mABs Permanent
NHIA sent a letter to HHS requesting that the agency make permanent Medicare payment for COVID-19 monoclonal antibodies (mABs).
STAY INFORMED. VISIT NHIA.ORG/COVID19 FOR INFORMATION AND RESOURCES ABOUT CORONAVIRUS DISEASE (COVID-19).

NHIA sent a letter to HHS requesting that the agency make permanent Medicare payment for COVID-19 monoclonal antibodies (mABs).
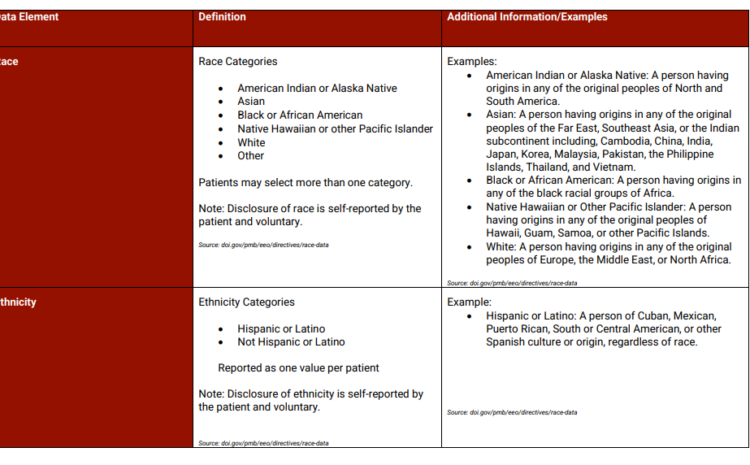
NHIF has adopted the Office of Management and Budget and Health and Human Services standard categories for race and ethnicity and urges all providers to include voluntary questions in their admissions process to capture this data.

On April 19, 2022, Baxter announced a supply disruption of the ExactaMix valve sets (ExactaMix 1200 Valve Set H938792 and ExactaMix 2400 Valve Set H938724) due to raw material constraints that are impacting production volumes. As a result, healthcare providers and institutions are driven to consider conservation measures when compounding using the ExactaMix Automated Compounder.

NHIA submitted comments on California SB 958, the Medication and Patient Safety Act of 2022, supporting amendments to the proposed legislation that allow for infused medications to be administered in the enrollee’s home when the physician and patient determine it is in the patient’s best interest and weighing in on various other aspects of the bill.
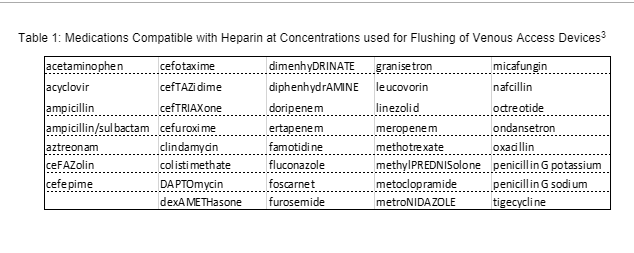
NHIA has compiled product shortage recommendations to help clinicians manage patient care and conserve prefilled NS syringe supplies during this time when they may not be readily available.
The National Home Infusion Foundation (NHIF) is proud to announce the publication of the first issue of Infusion Journal. The new scholarly journal features independent research and studies related to infused therapies, patient outcomes, medication safety, economic analyses, and case studies.



The National Home Infusion Foundation (NHIF) is proud to announce that Mitra Gavgani, PharmD has joined its Board of Directors for the 2022-2023 term.


NHIA submitted comments on a CMS proposed rule: Medicare and Medicaid Programs; CY 2023 Policy and Technical Changes to the Medicare Advantage and Medicare Prescription Drug Benefit Programs, which would alter the way pharmacy price concessions, including direct and indirect remuneration (DIR fees), are calculated by pharmacy benefit managers (PBMs).


The National Home Infusion Association (NHIA) is proud to announce that Erick Siegenthaler, PharmD, MHA, has joined the association’s Board of Directors for a 3-year term.


View photos from NHIA’s 2022 Annual Conference held in Nashville, Tennessee March 12-16.


More than 1,200 home infusion therapy professionals and 110 companies supplying products and services to the industry attended educational and networking events as well as a trade Expo.


The National Home Infusion Foundation (NHIF) is proud to announce that the recipient of the 2022 Outstanding Abstract Achievement Award (OAAA) is Cheyenne D. Johnson, PharmD from Option Care Health.


The National Home Infusion Foundation (NHIF) announces the finalists for its the Outstanding Abstract Achievement Award for 2022.


The National Home Infusion Association (NHIA) is pleased to announce the 2022 Fellow Program (FNHIA) cohort, made up of highly accomplished home and alternate site infusion professionals. The program is administered and funded by the National Home Infusion Foundation (NHIF), thanks to a generous contribution from Integrated Medical Systems, Inc. (IMS), the 2022 sponsor of the NHIA Fellow Program.


The National Home Infusion Foundation (NHIF) is proud to announce that Melissa Leone, RN, BSN is the recipient of the 2022 Lynn Giglione Women in Leadership Award.


The National Home Infusion Association (NHIA) is proud to announce that Dr. Sohail Masood, PharmD is the recipient of the 2022 Gene Graves Lifetime Achievement Award.
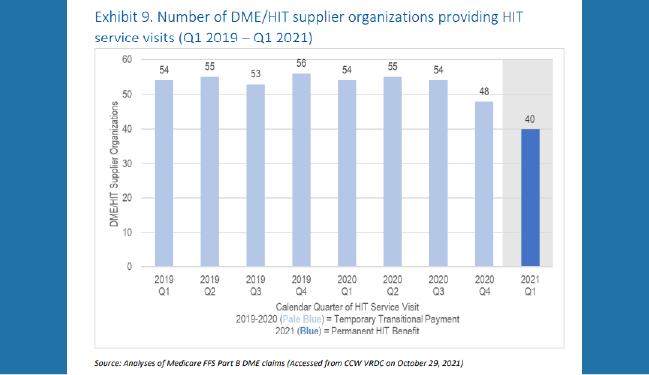

CMS recently issued a report which summarizes utilization trends for the Medicare Part B home infusion therapy (HIT) services benefit indicating the benefit has failed to draw sufficient participation from providers to ensure equitable access to beneficiaries across the U.S.
NHIA sent a letter to HHS requesting that the agency make permanent Medicare payment for COVID-19 monoclonal antibodies (mABs).
NHIF has adopted the Office of Management and Budget and Health and Human Services standard categories for race and ethnicity and urges all providers to include voluntary questions in their admissions process to capture this data.
On April 19, 2022, Baxter announced a supply disruption of the ExactaMix valve sets (ExactaMix 1200 Valve Set H938792 and ExactaMix 2400 Valve Set H938724) due to raw material constraints that are impacting production volumes. As a result, healthcare providers and institutions are driven to consider conservation measures when compounding using the ExactaMix Automated Compounder.
NHIA submitted comments on California SB 958, the Medication and Patient Safety Act of 2022, supporting amendments to the proposed legislation that allow for infused medications to be administered in the enrollee’s home when the physician and patient determine it is in the patient’s best interest and weighing in on various other aspects of the bill.
NHIA has compiled product shortage recommendations to help clinicians manage patient care and conserve prefilled NS syringe supplies during this time when they may not be readily available.
The National Home Infusion Foundation (NHIF) is proud to announce the publication of the first issue of Infusion Journal. The new scholarly journal features independent research and studies related to infused therapies, patient outcomes, medication safety, economic analyses, and case studies.
The National Home Infusion Foundation (NHIF) is proud to announce that Mitra Gavgani, PharmD has joined its Board of Directors for the 2022-2023 term.
NHIA submitted comments on a CMS proposed rule: Medicare and Medicaid Programs; CY 2023 Policy and Technical Changes to the Medicare Advantage and Medicare Prescription Drug Benefit Programs, which would alter the way pharmacy price concessions, including direct and indirect remuneration (DIR fees), are calculated by pharmacy benefit managers (PBMs).
The National Home Infusion Association (NHIA) is proud to announce that Erick Siegenthaler, PharmD, MHA, has joined the association’s Board of Directors for a 3-year term.
View photos from NHIA’s 2022 Annual Conference held in Nashville, Tennessee March 12-16.
More than 1,200 home infusion therapy professionals and 110 companies supplying products and services to the industry attended educational and networking events as well as a trade Expo.
The National Home Infusion Foundation (NHIF) is proud to announce that the recipient of the 2022 Outstanding Abstract Achievement Award (OAAA) is Cheyenne D. Johnson, PharmD from Option Care Health.
The National Home Infusion Foundation (NHIF) announces the finalists for its the Outstanding Abstract Achievement Award for 2022.
The National Home Infusion Association (NHIA) is pleased to announce the 2022 Fellow Program (FNHIA) cohort, made up of highly accomplished home and alternate site infusion professionals. The program is administered and funded by the National Home Infusion Foundation (NHIF), thanks to a generous contribution from Integrated Medical Systems, Inc. (IMS), the 2022 sponsor of the NHIA Fellow Program.
The National Home Infusion Foundation (NHIF) is proud to announce that Melissa Leone, RN, BSN is the recipient of the 2022 Lynn Giglione Women in Leadership Award.
The National Home Infusion Association (NHIA) is proud to announce that Dr. Sohail Masood, PharmD is the recipient of the 2022 Gene Graves Lifetime Achievement Award.
CMS recently issued a report which summarizes utilization trends for the Medicare Part B home infusion therapy (HIT) services benefit indicating the benefit has failed to draw sufficient participation from providers to ensure equitable access to beneficiaries across the U.S.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |